12 August 2021
Dr David Cutress: IBERS, Aberystwyth University.
- Fluke parasite infections cause a significant economic burden on agriculture worldwide as well as negatively impacting animal health and wellbeing
- Understanding where and when fluke and their snail intermediate hosts are present across farms and individual fields could help target their control
- Several control strategies could be assisted via accurate mapping, improving the efficiency and reducing the costs associated with implementing these on farms
Fluke
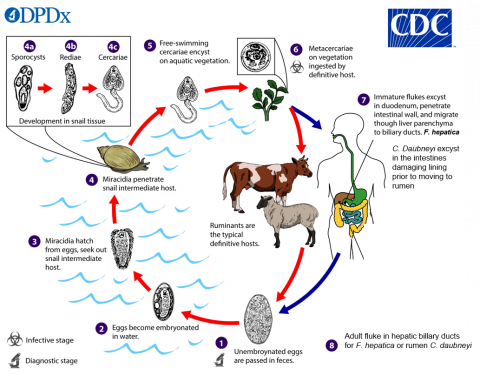
Within ruminant production systems, many health concerns can have a significant impact on livestock welfare and production efficiencies. One particular area of concern involves the impacts on animal health and welfare associated with parasitic infections. Whilst an article has recently discussed the impacts of gastrointestinal nematodes (GINs), another group of parasites familiar to farmers will be fluke worms (part of the trematode class). The two most discussed species, where UK agriculture is concerned, are the liver fluke (Fasciola hepatica) and the rumen fluke (Calicophoron daubneyi), which as their names suggest target the liver and the rumen respectively. Both species follow a similar life cycle that involves transmission through an ‘intermediate’ animal before infection of the livestock animals themselves. It has been suggested that impacts of liver fluke within the cattle industry in the UK may be as high as £23 million annually, whilst sheep losses are noted in the region of £3 - £5 per infected sheep and global livestock production losses may be €2.5 billion. Furthermore, there is increasing evidence of resistances developing to anthelmintic treatments which could increase production losses, treatment costs and control costs. Whilst clearly an economic burden, these parasites are also detrimental to animal wellbeing and due to production losses, they indirectly impact the environmental sustainability of red meat and dairy production systems worldwide. This is a particular issue as the sector strives to move towards sustainability and net zero-emission targets.
In the UK the intermediate animal that flukes rely on is a mud dwelling snail known as Galba truncatula, a very small snail species which is difficult to see by eye in its preferred habitats of small bodies of water (≥1m2) such as ditches (natural or even from tractor tyres) and other humid areas with open water and aquatic vegetation. Knowing an area of a farm has an active presence of Galba snails could be a great tool for understanding potential parasite transmission routes. As such strategies to identify likely habitats, presence of snails and density of snails is one route of research. The size of snails and the scope of livestock fields makes finding snails by eye a daunting and highly labour-intensive prospect, particularly when considering other similar mud species snails exist which could confuse results. Previous research has attempted to use geographic information systems (GIS) and remote sensing tools to identify likely snail habitats via satellite imagery which could help to narrow down the search. Another promising area of research is the investigation of species present within an environment based on finding their DNA. This environmental DNA (eDNA) approach has been undergoing development where Galba snails, as well as the parasites themselves, are concerned within IBERS Aberystwyth University and has led to previous and current collaborations with Farming Connect and European Innovation Partnership (EIP) Wales.
Galba truncatula snail left-hand picture courtesy of PreciseAg
Environmental DNA (eDNA)
The term eDNA refers to genetic material (nucleic acids) present in reservoirs in the environment including water, air, sediment, whole cells, extracellular DNA and whole organisms. The ability for DNA to last within these reservoirs allows evaluation of which organisms may have been present and is being increasingly used to replace visual observation and trap and release analysis when performing biological surveys. As DNA can rarely survive in the environment for too long (unless certain conditions regarding temperature and UV exposure are met) collected DNA acts as a good indication of species present in a relatively recent timeframe (most eDNA being degraded in 10 days). Combining eDNA collection from environments with modern polymerase chain reaction (PCR) techniques can allow a specific organism’s (or a group of organisms via multiplex assays) presence to be tested rapidly and quantitatively to assess the number of organisms present. Parallel research has been performed which confirms the use of this tool for both the snail hosts and the parasites themselves in Wales and Australia, demonstrating its wide global concern. As such this could act as a promising tool for sampling possible snail/fluke risk areas on farms during key times of the year towards developing bespoke farm level habitat risk maps. Though further research to determine snail and parasite eDNA shedding rates (particularly at different life cycle stages of the parasite) and relating eDNA levels with organism abundance in future could improve risk mapping even further.
Habitat risk mapping
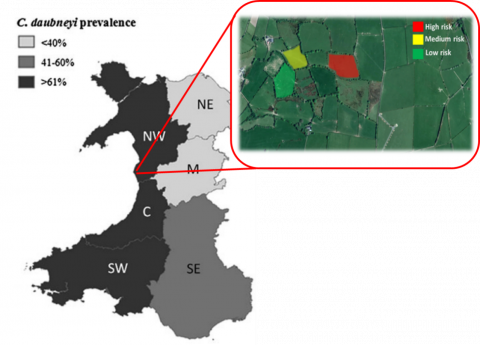
Habitat risk mapping to highlight areas of concern on a per field per farm basis could be a beneficial task to perform. As noted satellite imagery has attempted to be utilised to do this in a remote automated way but farmers tend to have intimate knowledge of their fields that could be used (particularly useful for areas under tree cover which satellites would be unable to assess). Systems based on previous research of weather and climate conditions on parasite infection rates have allowed the production of broad risk systems such as NADIS’ parasite forecast or GLOWORM-FL (aimed specifically at nematodes). Such systems can give reports to regional areas (suggested per 40km2) but it is well known that within an area infected and non-infected farms can be found adjacent to each other suggesting the need for higher resolution than 40km2. The resolution of mapping possible via eDNA methodology is essentially only limited by the number and level of samples taken across a field or farm but could effectively give m2 level accuracies.
Adaptation from Jones et al., (2017) demonstrating C. daubneyi presence at individual field level
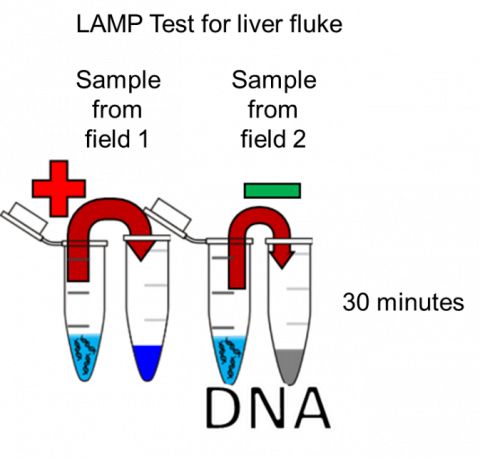
The current PCR methodologies which have been validated and discussed above still require samples to be collected and sent for processing in labs, realistically taking around half a day to a day for results to be available. However, there is scope in using developing DNA amplification techniques such as Loop-mediated isothermal amplification (LAMP) which could allow on-farm testing in as little as 30 minutes. LAMP has already been validated for use in milk for mastitis pathogen detection as well as poultry pathogen detection suggesting it may be a developing future diagnostic tool for farms. Other than the speed of results with LAMP one of its key benefits as a diagnostic tool is its ability to easily be linked to DNA binding dyes or metal ion indicators to provide a visible change that can be seen by eye (or by smartphone) and requires no specialist expensive equipment. With more optimisation, it is possible to refine this tool to be a field-based diagnostic kit that farmers could perform themselves.
Control strategies
Fasciolosis is a disease caused by liver fluke and it has a range of clinical and sub-clinical effects. As the juvenile fluke migrates through the intestinal wall, the main symptoms are dyspepsia (bloating/discomfort) and ascites (fluid build-up in the abdomen). As fluke mature and reach the liver symptoms are general immune-inflammatory linked responses such as fever, hepatomegaly, splenomegaly and anaemia. For sheep farmers, this presents as chronic/sub-acute symptoms like weight loss, body condition loss, poor fleece quality, ventral oedema (bottle jaw), lethargy, or acute symptoms such as reduced grazing and sudden deaths. Cattle, on the other hand, require far higher chronic infection levels to present with reduced milk yields, poor fertility, excessive weight loss, birthing weak calves and chronic diarrhoea. C. daubneyi infection of ruminants causes paramphistomosis suggested to cause livestock morbidity, and symptoms including; scour, lethargy, submandibular oedema and dehydration during the immature parasite stages. Whilst adverse effects have been documented for the acute immature stage of infection, it is unclear to date if there are significant negative effects to sub-clinical chronic infection, though recent studies of rumen related genes, extracellular vesicles and proteins suggest possible increased acidosis occurrences as rumen fluke burden increases. Based on these impacts the development and optimisation of control strategies are a necessity. Signs of disease, particularly where persistent, should also be investigated by a veterinary surgeon to ensure the correct diagnosis and treatment. Discussions with vets and other agricultural specialists can also be key to developing and maximising the effect of control strategies put in place alongside prescribed treatments.
In the UK COWS (Control of Worms Sustainably) and SCOPS (Sustainable Control of Parasites in Sheep) offer a wealth of information for farmers towards controlling parasites and dealing with drug resistance developments. Other than drugs, TST and refugia population control, strategies can include: correct quarantine of sick or imported livestock, avoidance of grazing risk fields (muddy, wet fields or fields where known infected animals have recently been), rotational grazing to break parasite life cycles within fields and mixed-species grazing. The alternate route to targeting parasites themselves is to target the intermediate snail host as a control mechanism. Whilst molluscicide chemicals exist these would likely have adverse impacts on other native snail species alongside ecosystems and environmental concerns generally. Natural plant compounds could be another route of snail control which are currently undergoing research. More simply, however, reducing the physical proximity overlap of snails with livestock animals can be a method to reduce the transmission of parasites between hosts. Utilising mapping tools above strategic fencing and drainage of areas known to have active populations of either snail intermediate hosts or the parasites themselves can help to target infrastructure applications and reduce costs. For example, if a corner of a known wet field is found to have snails this can be specifically fenced (or temporarily electric fenced) off rather than removing the grazing of the entire field. Risk mapping of fields could also combine well with determining when animals should be faecal egg counted or treated, for example after rotational grazing into medium risk fields or past known high-risk fields.
Summary
Liver and rumen fluke pose a significant risk to farmers globally and across the UK concerning economic losses and animal welfare. With evidence of increasing infection risk patterns and higher prevalence levels potentially linked to climate change, it is ever more important that new diagnosis and control strategies combat these. A relatively new development is the ability to screen environments using eDNA, this may offer a unique opportunity to map infection risk down to specific field levels within farm environments. Initial work suggests flukes, and more specifically their intermediate hosts, offer a good potential target for identification using eDNA. Testing utilising eDNA could provide positive negative identification of snail/parasite risk areas and could even in the future be used to provide quantification of the level of infection risks. LAMP, a DNA amplification technology, could allow rapid, visual and relatively simple analysis to be performed on farms rather than having to process samples in laboratory environments. Mapping risks could enable farmers to accurately implement informed strategies to reduce livestock and snail/parasite interactions to reduce transmission rates.
If you would like a PDF version of the article, please contact heledd.george@menterabusnes.co.uk